WHY
- To check and compare the in vivo performances of your prototypes
- To demonstrate the general and local safety of your implantable device in accute phase and long-term period
- To assess the efficacy of a new therapy relatively to gold-standard treatment
The justification of a preclinical investigation may be Regulatory Filing, Upstream Research or a need for Medical and Scientific Information.
WHAT
- In-vitro proliferation assays on drugs and biomaterials in animal and human cancer cell lines, fibroblasts, endothelial cells
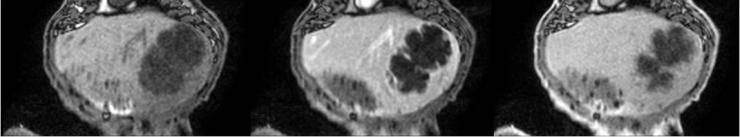
- Large in-vivo models of healthy tissues, vascular, inflammatory and cancer disease: healthy models of vascular embolization in sheep, swine and rabbits, subcutaneous implantation tests, hypervascular VX2 tumors in rabbits
- Molecular biology: ELISA and Western blot protein quantification in tissues samples, cDNA microarrays for gene expression in all animal species
- Pathology: conventional staining (HES, Masson, orcein, Sirius red, Perls...), digitization of histology sections, image analysis and morphometric measurements, ground sectioning of hard samples, immunohistochemistry and in situ hybridization for microvascular density, cell proliferation, inflammatory cells
- Access to Imaging Plateforms: 3D Angiography (see animation), MR Imaging of tumor in vivo / ex vivo (T1, T2, DWI, Gd enhanced…), Ultrasound follow-up of tumor volume
- Physical and chemical testings: compression tests, granulometric analysis, catheter injectability, in vitro resorption, chemical imaging of devices and tissues (infrared, Raman and fluorescence microspectroscopies
- Pharmacology: Plasma and tissue dosage of antineoplastic drug, PK analysis
- Data analysis: parametric, non-parametric, multivariate statistical analysis
WHO
Procedures are performed by KOLs with long experience in preclinical models. Analyses are performed in collaborations with Academic and Private Labs under the supervision of the Management Team and of Institutional Experts.
HOW
Assignment for a single Service with delivery of raw data or for a complete Protocol from Study Design to Study Report and Scientific Communication
The experimentation facility is certified EN ISO 9001 and EN ISO 13485 by G-Med.
Archimmed has experience in conducting GLP compliant Protocols.
All protocols are reviewed by the local Ethics Committee (COMETHEA) and get approval of the Ministère de l'Enseignement Supérieur et de la Recherche according to the European Directive 2010/63/EU and its transposition in French Law.
Archimmed has received accreditation for conducting R&D Projects eligible for Credit D'impot Recherche by the French Ministry of Education and Research.